Introduction: Venetoclax (Ven) has changed the chronic lymphocytic leukemia (CLL) treatment landscape with the ability to produce deep responses that result in improved long-term outcomes with well-tolerated fixed-duration regimens. Due to its potent pro-apoptotic effect on CLL cells, Ven poses a risk of inducing tumor lysis syndrome (TLS). If not managed promtly and appropriately, metabolic abnormalities (laboratory TLS) can progress to clinical TLS, including renal insufficiency, cardiac arrhythmias, seizures, and death. Current Ven TLS mitigation measures include TLS risk assessment, 5-wk dose ramp-up, prophylaxis with hydration and oral uric acid reducers, and close monitoring of blood chemistry based on risk level (Davids. Clin Cancer Res. 2018;24:4371). Debulking strategies have been effectively used to lower TLS risk before Ven therapy. In this analysis, rates of TLS and outcomes in the clinical trial and post-marketing settings are evaluated.
Methods: Trial data from patients treated with current TLS mitigation measures implemented since May 2014 were pooled from 8 studies (N=1138; n=304 treatment naïve; n=834 relapsed/refractory [R/R]). This was a retrospective descriptive analysis of adverse events (AEs) of TLS and cases meeting laboratory and clinical TLS using Howard criteria (Howard. N Engl J Med. 2011;364:1844). Post-marketing data included reports of TLS from the AbbVie Global Safety Database (GSD) collected from Apr 2016 - Dec 2019 (N=236) and 5 publications of real-world usage (n=817 patients over all studies). The review of trial data focused on Ven ramp-up (ie, first 5 weeks of dosing).
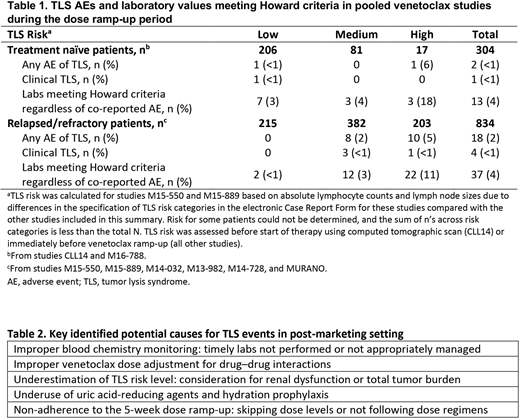
Results: In 1138 patients receiving Ven from pooled clinical studies, AEs of TLS and laboratory values meeting Howard criteria are shown in Table 1. With current recommended mitigation measures, the incidence of TLS AEs was 1.8% (n=20), with 15 laboratory TLS AEs (1.3%) and 5 clinical TLS AEs (0.4%) reported. TLS was transient in all cases, and patients resumed therapy without permanent sequelae. With the currently recommended measures, no deaths from TLS were reported in the 1138 patients in clinical trials.
Approximately 20,000 patients with CLL have initiated Ven outside of clinical trials from 2016-2019 based on commercial tracking. In this post-marketing setting, 236 reports of TLS were identified in the GSD, and 17 had severe consequences that could be attributed to TLS (13 fatal, 4 requiring dialysis), all in patients with R/R CLL. These patients had typical demographic characteristics of CLL, with a median age of 69 years and multiple comorbidities, where reported, including 7 patients with renal dysfunction. Ten patients (59%) were hospitalized before receiving Ven. In those with available data, TLS occurred in patients with high (n=3), medium (n=6), and low (n=1) TLS risk. TLS mostly occurred after the 20-mg dose (n=8) but also after the 50- (n=1), 100- (n=6), and 400-mg (n=1) doses. Noting the incomplete data available, blood monitoring and interventions were not reported in most patients; recommended dose ramp-up was reportedly not followed in 4 cases. Concomitant anti-CLL therapies or contraindicated therapies (CYP3A inhibitors) were used in 3 and 2 cases, respectively. Key factors contributing to TLS in the post-marketing setting are summarized in Table 2.
Five real-world studies, mostly in patients with R/R CLL, reported on the incidence of TLS (n=817 patients; VeRVe observational study; Mato. Haematologica. 2018;103:1511; Clin Cancer Res. 2019;25:4264; Kohler. Leuk Lymphoma. 2020; Nabhan. J Clin Oncol. 2018;36[suppl, abstr]:7529). TLS incidence ranged from 6%-13% (clinical TLS, 1%-6%). Reported TLS risk distribution ranged from 38%-45% (low), 34%-37% (medium), and 19%-28% (high).
Conclusion: This report's findings indicate that TLS mitigation measures implemented in clinical studies (N=1138) successfully mitigated the risk of TLS with Ven in patients with CLL, with clinical TLS occurring in <1% with no irreversible sequela. For clinical TLS in the post-marketing setting, it is important for healthcare professionals to follow recommended preventive measures, particularly applying appropriate risk assessment, prophylaxis and dose ramp-up, blood chemistry monitoring and early intervention, and dose modifications for drug interactions (Table 2).
Seymour:Nurix: Honoraria; Morphosys: Consultancy, Honoraria; Mei Pharma: Consultancy, Honoraria; Gilead: Consultancy; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Gribben:Janssen: Honoraria, Research Funding; Celgene: Research Funding; Abbvie: Honoraria; AstraZeneca: Honoraria, Research Funding. Davids:Sunesis: Consultancy; Research to Practice: Honoraria; Zentalis: Consultancy; Syros Pharmaceuticals: Consultancy; Novartis: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Surface Oncology: Research Funding; Genentech: Consultancy, Research Funding; AbbVie: Consultancy; Ascentage Pharma: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Eli Lilly: Consultancy; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Celgene: Consultancy; BeiGene: Consultancy; Adaptive Biotechnologies: Consultancy; Gilead Sciences: Consultancy; Merck: Consultancy; Bristol Myers Squibb: Research Funding; Janssen: Consultancy. Mato:Loxo: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Sharman:AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; BeiGene: Research Funding; Acerta: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Cyr:AbbVie: Current Employment, Current equity holder in publicly-traded company. Bartkus:AbbVie: Current Employment, Current equity holder in publicly-traded company. Pena:AbbVie: Current Employment, Current equity holder in publicly-traded company. Boyer:Roche: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Sharmokh:AbbVie: Current Employment, Current equity holder in publicly-traded company. Rosenberg:AbbVie: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal